
Your skills turn a treatment into her cure.
Enable extraordinary moments.
Research Nurse III, RN, Clinical Trials
At Kaiser Permanente our nurses are leaders, clinicians, researchers, innovators, and scientists who are contributing toward being an industry-leading voice for advancing evidence-based care. Whether supporting the patient directly in our hospitals or clinics, providing care at home, serving our patients through innovative virtual technology, or managing care delivery teams, Kaiser Permanente nurses utilize scientific evidence and our integrated care model to optimize the total health of our members and the communities we serve. We invite nurses who are passionate about nursing excellence, high-quality compassionate care delivery, professionalism, integrity, teamwork and patient and family centeredness to join our teams so that we can continue to sustain and build upon our culture of excellence.
-
Job Schedule: Full-time
-
Scheduled Weekly Hours:
-
Shift: Day
Success Profile
We’re looking for Nurse Leaders who possess the following traits.
- Collaborative
- Compassionate
- Flexible
- Leadership
- Socially Conscientious
- Trustworthy
Benefits
We offer several benefits to our nurses.
Our Culture
At Kaiser Permanente, we cultivate an environment of compassion, integrity, trust, and open communication that helps our teams do their best work. We believe that lifelong learning will expand our knowledge so we can better serve our patients. Our practice is rooted in research and evidence-based care. Our nurses reflect the rich diversity of our members and communities and provide culturally responsive and competent care that promotes understanding of our members needs and preferences. At Kaiser Permanente, nurses are highly skilled professionals who exemplify leadership, critical thinking, and collaborative problem solving and deliver the right care, at the right time, in the right setting.
Hear From Our People

Caitlin
Inpatient Lactation Consultant
As an RN/IBCLC in Maternal Child, I’m meeting families at a pivotal time — I’m there to help launch a new family into the world. Everything I do has the potential to impact a family’s health for generations to come.

Kimberly
Quality Coordinator RN
In Hawaii, your community is your family — or ‘ohana.’ I am able to provide extraordinary patient-centered care to my ohana.

David
Appointment and Advice RN
The location is fantastic. Kaiser Permanente is all over the country. You can pick almost any environment you want — from ocean to mountain to city. You’ll still be able to contribute to our mission of providing high quality health care at a local facility.

Shawn
BSN, RN
I chose Kaiser Permanente because their staff and providers really show that they care about you. Also, throughout my 12 years of working here I’m always learning something new, and I’ve had many opportunities for growth and development.

Kristen
Relief Charge Nurse
Working as a nurse at Kaiser Permanente has allowed me the perfect flexible schedule so that I can volunteer as Girl Scout Leader and room parent for both my children. My kids know that I am a hardworking mother who can provide, as well as a mom who can be present and be there for them – and that means everything to me.

Jazmin
RN, Advice Nurse
Working for Kaiser Permanente has allowed me to take control of my future and make my goals a possibility. I feel that Kaiser Permanente allows me to keep climbing and moving. Having that ability truly makes me feel empowered.
Research Nurse III, RN, Clinical Trials
In addition to the responsibilities listed below, this position is also responsible for leveraging comprehensive foundational clinical research knowledge in all aspects of nursing practice; participating in the implementation of new protocols; administering standard and nonstandard research interventions; evaluating the patients response to therapy; responding to variances in protocol implementation and reporting variances to the research team; integrating evidence-based practice into nursing practice and evaluating patient outcomes; providing nursing care and judgement of research activities within scope of practice; communicating the impact of the research process on patient care; adjusting interventions based on findings; collaborating with the interdisciplinary team and institutional leaders on improvement activities related to promoting patient/participant safety, clinical quality and reducing risk; and implementing quality improvement activities in the team or program of care level.
- Pursues effective relationships with others by proactively providing resources, information, advice, and expertise with coworkers and members. Listens to, seeks, and addresses performance feedback; provides mentoring to team members. Pursues self-development; creates plans and takes action to capitalize on strengths and develop weaknesses; influences others through technical explanations and examples. Adapts to and learns from change, challenges, and feedback; demonstrates flexibility in approaches to work; helps others adapt to new tasks and processes. Supports and responds to the needs of others to support a business outcome.
- Completes work assignments autonomously by applying up-to-date expertise in subject area to generate creative solutions; ensures all procedures and policies are followed; leverages an understanding of data and resources to support projects or initiatives. Collaborates cross-functionally to solve business problems; escalates issues or risks as appropriate; communicates progress and information. Supports, identifies, and monitors priorities, deadlines, and expectations. Identifies, speaks up, and implements ways to address improvement opportunities for team.
- Works on budgeting and financing by: developing a working understanding of how to provide input on implementing budget components for internally funded standard and nonstandard projects.
- Conducts clinical research by: independently collecting data in clinical trials (e.g., recruiting human subjects, administering surveys and/experiments); leveraging a comprehensive foundational knowledge of source data and the tools leveraged for analyzing, and interpreting clinical data; leveraging a comprehensive foundational knowledge of clinical trials, studies, and interventions at the site-level; independently contributing to the development of standard and nonstandard clinical research protocols and other processes of clinical trials; preparing and/or supporting the submission of study documentation to regulatory bodies (e.g., IRB) for review and approval prior to implementation; may also be responsible for executing standard and non-standard clinical trial activities (e.g., informed consent process, lab support and processing, pharmaceutical documentation data, sample processing, adverse event assessment process); and may be involved in supporting standard and non-standard research lab operations(e.g., biological specimen collection, including patient preparation, labeling, handling, preservation or fixation, processing or preparation, and transportation and storage of specimens).
- Ensures research compliance by: independently drafting and/or preparing and submitting clinical trial applications in compliance and consistency with all applicable federal, state, and local regulations, and reviewing and providing input on KP compliance policies and procedures; identifying compliance and/or quality issues and assisting in the development of corrective action plans, escalating as necessary; identifying opportunities to update compliance-monitoring/audit systems and documentation; utilizing standard approaches to analyze risk-management data and making recommendations to mitigate potential risk; independently implementing research protocols, procedures, and guidance to ensure confidentiality, privacy, and security of clinical research data; and leveraging comprehensive research expertise to provide guidance to investigators and other key stakeholders to ensure compliance with IRB approved protocols and local and federal guidelines.
- Maintains internal and external effective working relationships by: leveraging a comprehensive foundational knowledge of how to partner with research stakeholders and investigators within and across units to contribute to research projects; independently communicating with key contractors and subcontractors, study sponsors, collaborating internal and/or external clinical sites, and data coordinating centers as applicable; and developing materials for supporting the education of staff and/or participants on protocols, documentation procedures, clinical best practices, or timeliness of submissions with limited guidance.
- Ensures documentation of clinical research files by: completing documentation of all research activities (e.g., consent forms, reports, tracking forms) in a timely and accurate manner; assisting with the development and/or implementation of standard and nonstandard quality control and/or assurance measures and documenting feedback for the research staff and management; assessing volunteers and/or patients for eligibility to participate in Clinical Trials using standard procedures and criteria; and leveraging a comprehensive foundational knowledge of how to monitor and audit Clinical Trials as well as documenting findings.
- Bachelors degree in Nursing AND minimum two (2) years of experience in clinical health care or a directly related OR Associates degree in Nursing AND minimum four (4) years of experience in clinical health care or a directly related field.
- Registered Nurse License (California) required at hire
- Knowledge, Skills, and Abilities (KSAs): Employee/Labor Relations; Research Databases; Regulatory Agencies; Nursing Principles; Health Care Operations; Ethical Conduct; Clinical Research Quality; Innovative Mindset; Data Quality; Business Relationship Management; Managing Diverse Relationships; Stakeholder Management; Project Management Tools; Quality Assurance Process; Quantitative Research Methods; Survey Methodology; The Scientific Method; Computer Literacy; Accountability; Adaptability; Autonomy; Organizational Skills; Compliance; Clinical Research; Laboratory Procedures; Laboratory Equipment; Project Management; Experimental Design
- Certified Clinical Research Professional (CCRP) from Society of Clinical Research Associates (SoCRA); OR ACRP Certified Professional, Certified Clinical Research Associate, Certified Clinical Research Coordinator, Certified Principal Investigator, ACRP Medical Device Professional, or ACRP Project Manager from Association of Clinical Research Professionals (ACRP); OR Certified in Healthcare Compliance (CHC) from Healthcare Compliance Association (HCCA).
- Basic Life Support (BLS) Certification from the American Heart Association
Navigating the Hiring Process
We're here to support you!
Having trouble with your account or have questions on the hiring process?
Please visit the FAQ page on our website for assistance.
Need help with your computer and browser settings?
Please visit the Technical Information page for assistance or reach out to the web manager at kp-hires@kp.org.
Do you need a reasonable accommodation due to a disability?
Reasonable accommodations may be available to facilitate access to, or provide modifications to the following:
- Online Submissions
- Pre-Hire Assessments
- Interview Process
If you have a disability-related need for accommodation, please submit your accommodation request and someone will contact you.
-

Ranked among the nation's best
Every hospital that's part of the Kaiser Permanente system earned “high performing” recognition in the latest U.S. News and World Report's annual rankings. That means all 39 of our hospitals are among the top 20% in the nation.
-

Awarded for excellence
We're proud that so many of our hospitals have earned Magnet status. It shows our real commitment to outcomes, safety, quality, efficiency, and, most importantly, to our nurses.
-

Recognizing nurses during Nurses Month in 2022
We're thanking each one of our 65,000 Kaiser Permanente nurses for everything they've done for us and for the nation. Thank you for choosing to be a nurse and for leading our transformation of care.
-

Explore a career in nursing
When you join Kaiser Permanente as a nurse, you'll help us boldly transform care while also building a career that offers more opportunities for you to grow.


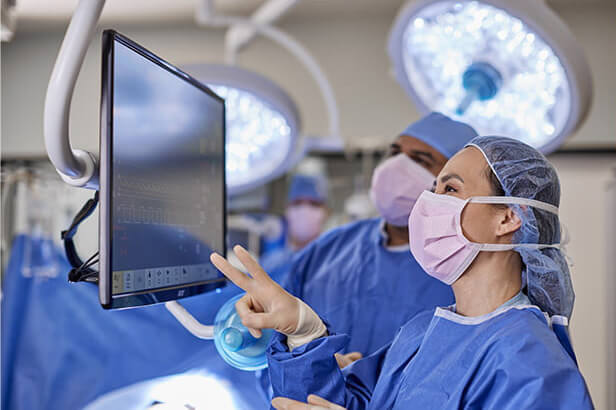

Experience nursing excellence
At Kaiser Permanente our nurses are leaders, clinicians, researchers, innovators, scientists, and more. It takes an extraordinary team to offer extraordinary care.
Our Events
Want to talk to a member of the Kaiser Permanente team? Meet us at an upcoming hiring event.
Jobs For You
You have no recently viewed jobs
You currently have no saved jobs

Join Our Talent Community
Join our Talent Network today to receive email notifications about our career opportunities that match your skills.






Connect With Us